Credits
Jonathan R. Hendricks, Alexandra Moore, and the authors (Robert J. Moye, Stephen F. Greb, and Jane A. Picconi) of the "Mineral Resources of the Southeastern US" chapter of The Teacher-Friendly Guide to the Earth Science of the Southeastern US, 2nd Edition (published in 2016 by the Paleontological Research Institution) from which portions of this chapter are extracted.
Chapter last updated: August 23, 2020
Chapter Images:
High-resolution versions of all images in this chapter are available for free download.
- Separate images (.zip; 4 MB)
- PowerPoint file (.pptx; 4.3 MB)
Contents
Page topics: Introduction; Atoms and Elements (Ions, Bonding, Ionic Bonding, Covalent Bonding, Bonding and Atomic Structure); Mineral Identification (Color, Luster, Hardness, Cleavage, Crystal Habit, Other Properties); Distinguishing Minerals from Gems; Formation of Mineral Deposits; Classification of Minerals
Image above: 3D photogrammetry models of a variety of different minerals. Image created by Jonathan R. Hendricks for the Earth@Home project (CC BY-NC-SA 4.0 license).
Introduction
How is a mineral different from a rock? A useful analogy is to think about a rock as a chocolate chip cookie, which is comprised of different ingredients: flour, eggs, white sugar, brown sugar, vanilla, and, of course, chocolate chips. Similarly, most rocks are comprised of multiple "ingredients" and these consist of different types of minerals.
The International Mineralogical Association recognizes approximately 5,500 different kinds of minerals. Minerals make up the rocks of the Earth’s lithosphere and mantle. They contain many of the nutrients in soil necessary for plant growth. They are crushed and processed to extract metals for all purposes and are also used in construction. Minerals that are used in manufacturing include silica for glass, kaolinite for ceramics, gypsum for wallboard (drywall), fluorite for the fluoride in toothpaste, montmorillonite clay for kitty litter, and halite (better known as common table salt and rock salt) to clear ice from roads. Some minerals are considered to be precious or semi-precious gemstones and are used in jewelry (learn more about gems below). Others are used to dye fabric and color paint. Metallic minerals have many applications in manufacturing. For example, iron comes from hematite and magnetite, and from it we make steel. Lead, from the mineral galena, is used in the manufacture of batteries and in the solder found in electronic devices. Titanium, from the mineral ilmenite, is used in airplanes, spacecraft, and even white nail polish. Aluminum comes from bauxite and is known for being both lightweight and strong—many of the parts that make up today's automobiles are made of this metal. Copper comes from a variety of copper-bearing minerals, including chalcopyrite, and is used to make electrical wire, tubing, and pipe.
In short, nearly every aspect of our lives—both biologically and socially—requires minerals. In fact, the average American uses, in one way or another, over 38,000 pounds of minerals every year (learn more).
Scientifically Speaking, What are Minerals?
To a geologist, a mineral is "a naturally occurring homogeneous solid with a definite ... chemical composition and a highly ordered atomic arrangement. It is usually formed by inorganic processes" (Klein and Hurlbut, 1999). What does this all mean?
- Naturally occurring: Not made by people.
- Homogeneous: One uniform compound.
- Solid: Not a gas or liquid.
- Definite chemical composition: Can be expressed by a chemical formula.
- Highly ordered atomic arrangement: There is a geometric relationship between the atoms that comprise the mineral.
- Inorganic processes: Not biological in origin and typically crystallizing from a liquid. (Note that this part of the definition is somewhat problematic, as some organisms use minerals such as calcite or aragonite to create their skeletons; the shells of clams and snails are examples.)
Because the structure and properties of minerals are defined by their underlying atomic composition (as is the case for all matter in the universe), a brief, simplified review of atomic-level chemistry is required.
In order to make use of minerals, we must first be able to identify them. This means understanding how minerals are formed, what they’re made out of, and how their constituent atoms and elements are put together. Earth scientists who identify and research minerals are called mineralogists.
Atoms and Elements: The Building Blocks of Minerals
Minerals, as well as well as every single thing that you will ever interact with during your life, are comprised of atoms, which are "small units of matter that combine in chemical reactions" (Press and Siever, 1994). It should be emphasized again that atoms are very, very small. You cannot see them, though—as shown in the video below—we can use sophisticated equipment to estimate what they look like.
"What Does an Atom Look Like?" by "What The Physics?!" (YouTube).
Atoms are comprised of three main components: protons, neutrons, and electrons. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge. As shown in the video above, the protons and neutrons in an atom are arranged in a nucleus that is surrounded by an electron cloud.
You may already be familiar with the periodic table of the elements shown below.
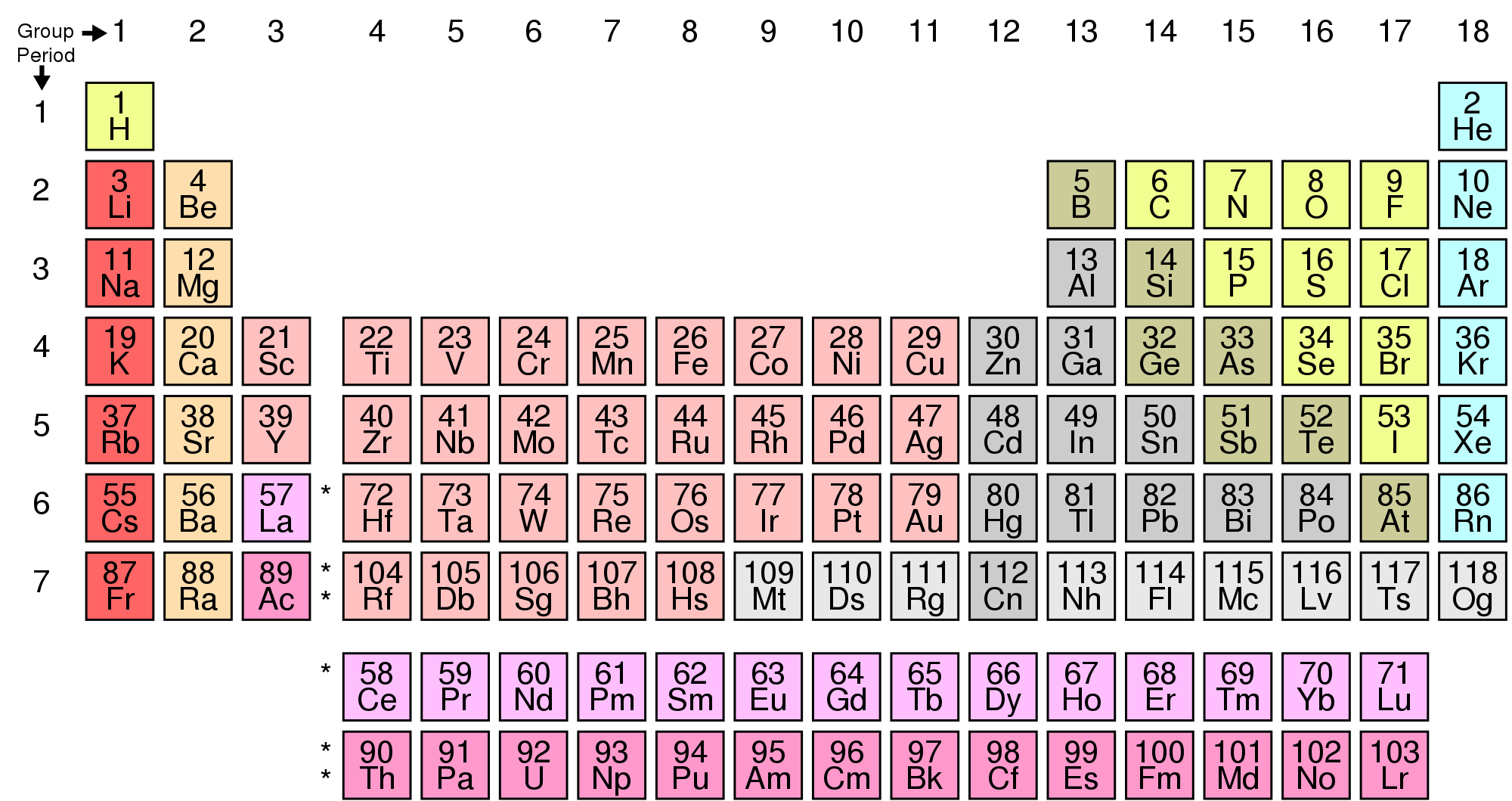
Periodic table of the elements; image by "Offnfopt" (Wikimedia Commons; public domain).
Each element on the periodic table is associated with a number that is called its atomic number (see image above). For example, the atomic number of hydrogen (H) is 1. The atomic number of gold (Au) is 79. The atomic number refers to the number of protons in the nucleus of a given atom, which is what defines each element. Helium (He) has a single proton in its nucleus, while gold has 79. If an atom had 80 protons in its nucleus, it would no longer be gold; it would instead be mercury (Hg).
Elements are the building blocks of minerals. The mineral quartz, for example, is made of the elements silicon and oxygen, and, in turn, is also a major component of many rocks. Most minerals present in nature are not composed of a single element, though there are exceptions such as gold. Elements such as copper (Cu), lead (Pb), zinc (Zn), and even silver (Ag), gold (Au), and carbon (C) in the form of diamond are not rare, but they are usually widely dispersed throughout rocks and occur at very low average concentrations. Eight elements make up (by weight) 99% of the Earth’s crust, with oxygen being the most abundant (46.4%). The remaining elements in the Earth's crust occur in very small amounts, some in concentrations of only a fraction of one percent. Because silicon (Si) and oxygen (O) are the most abundant elements in the crust by mass, it makes sense for silicate minerals like quartz and feldspar to be among the most common minerals in the Earth's crust.
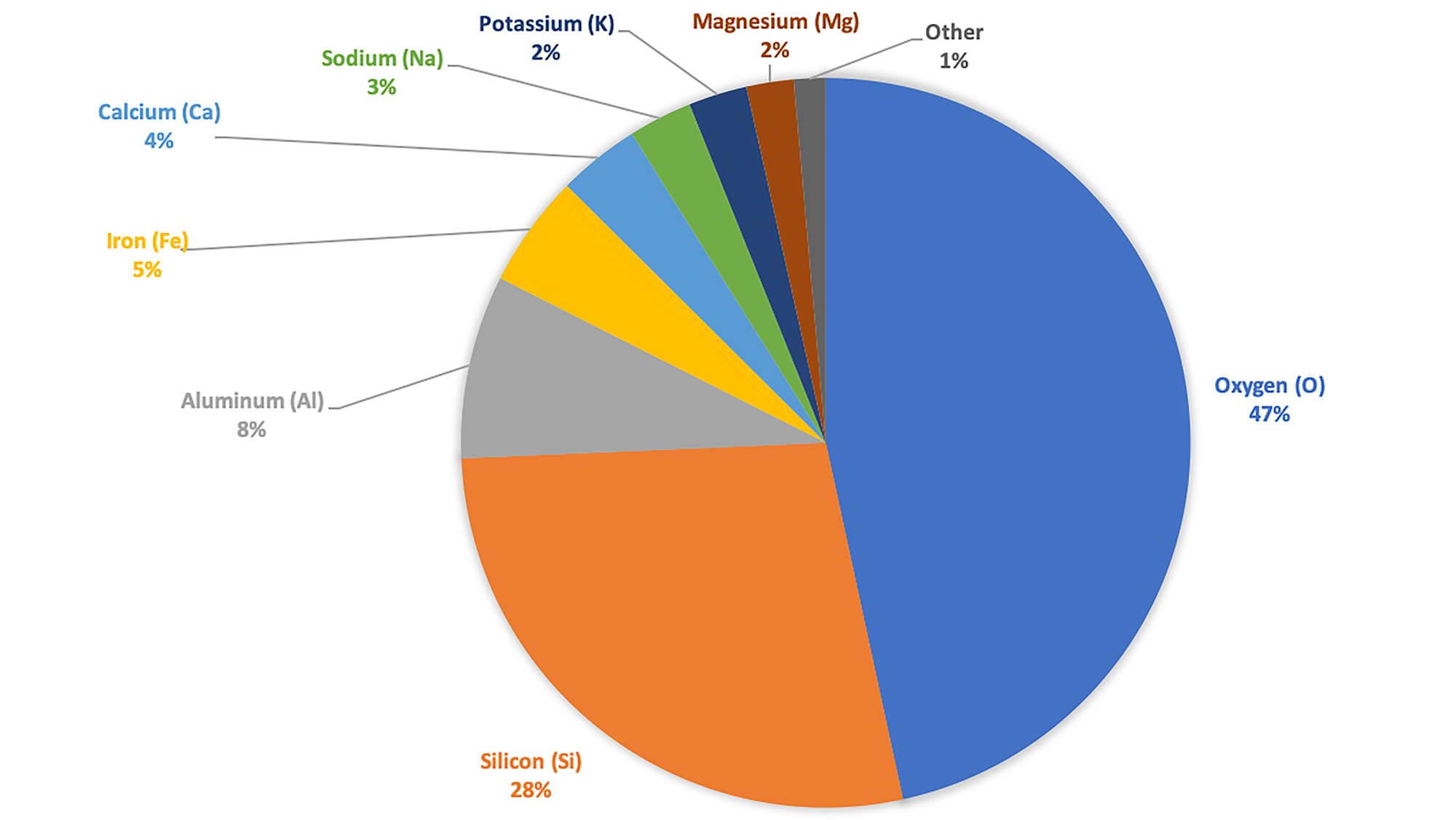
Weight percentages of different minerals in Earth's crust. Data are from Mason and Mason (1982), reproduced in Klein and Hurlbut, Jr. (1999). Image created by Jonathan R. Hendricks for the Earth@Home project (CC BY-NC-SA 4.0 license).
Let's now consider how atoms of different elements connect to form minerals.
Ions
Ions are atoms that have unequal numbers of protons and electrons. Atoms that have more protons than electrons have a positive charge and are called cations (for example, a cation of sodium is symbolized Na⁺). Anions are atoms that have more electrons than protons, resulting in a negative charge (for example, an anion of chlorine is symbolized Cl⁻). Atoms gain or lose electrons in order to become more energetically stable.
Bonding
Atoms join together to form larger structures by bonding. Two types of bonding are especially important for the building of minerals: ionic and covalent bonding.
Ionic Bonding
Approximately 90% of all minerals are built by ionic bonding, which results from exchange of electrons between atoms. The transfer of one or more electrons from one atom to another results in the formation of charged positive and negative ions (cations and anions, respectively). This in turn results in attraction between the two oppositely charged ions. Common table salt (or, halite) has the chemical formula NaCl and is built of two ions: Na⁺ (sodium) and Cl⁻ (cholorine).
"The formation of ionic bonds by sodium chloride" by Andy Todd (Sketchfab).
Covalent bonds
Covalent bonds result from two or more atoms sharing electrons (unlike in ionic bonds, electrons are not gained or lost). It is the strongest kind of chemical bond and is what gives the mineral diamond (pure carbon) its strength. Minerals with covalent bonds tend to be very hard, are insoluble (don't dissolve in water), and have a very high melting point.
Bonding and Atomic Structure
Most of the properties of a mineral (see below) are governed by its underlying atomic structure and the type(s) of bonding that connect the atoms within the mineral. While some minerals have only ionic (e.g., halite, or table salt) or covalent (e.g., diamond) bonding, many minerals have atoms that are connected by both types of bonds.
Mineralogists determine the underlying atomic structure of a mineral using an approach called x-ray diffraction, which is explained in the video below.
"What is X-ray Differaction" by Bruker Corporation (YouTube).
Mineral Identification
Although defined by their chemical composition and crystal structure, minerals are identified based on their physical properties. A variety of properties must usually be determined when identifying a mineral, with each such property eliminating possible alternatives. These include color, luster, hardness, cleavage, and density, as well as other properties.
Color
Minerals collectively represent all of the colors of the rainbow and more. Color plays a huge role in determining the value of precious gem stones, but is not a reliable diagnostic property for many types of minerals. For example, the common mineral quartz exhibits a great natural diversity of colors; indeed, amethyst is just purple-colored quartz.
"Amethyst Geode" by VirginiaTechUnivLibraries (Sketchfab).
Even though the color of a mineral might not be diagnostic for its identification, the color of the streak that it leaves behind when dragged across a white porcelain plate can be helpful. (Note that the streak plate has a hardness of 6.5, so minerals with Mohs hardness values above 6.5 will not leave a streak; learn more about mineral hardness below.) Doing so powderizes the mineral, eliminating factors like external weathering, crystal habit, impurities, etc. The resulting streak may be different in color from the specimen itself. Hematite, as shown in the video below, provides an excellent example of this.
"Mineral Streak Test A13a" by Scott Brande (YouTube).
Luster
Luster is the appearance that a mineral has as light reflects off of a fresh (non-weathered) surface. It is not the same as color. For example, quartz has a glassy appearance, but its color may vary significantly (clear, purple, yellow, etc.).
Metallic Luster
Description: Looks like metal.
Examples: Galena, pyrite, chalcopyrite, magnetite
Galena with metallic luster. Model by Nate Siddle (Sketchfab).
Adamantine Luster
Description: Brilliant
Example: Diamond, cerussite, and anglesite
Cut diamond (simulated; not a real specimen) with adamantine luster. Model by Miléd Hammoud (Sketchfab).
Vitreous Luster
Description: Glass-like appearance
Examples: Quartz and tourmaline
Tourmaline with vitreous luster. Model by Nate Siddle (Sketchfab).
Resinous Luster
Description: Resin-like (not as shiny as glass)
Examples: Sphalerite, sulfur
Sphalerite with resinous luster. Model by "rocksandminerals" (Sketchfab).
Pearly Luster
Description: Iridescent like mother-of-pearl.
Example: Muscovite
Muscovite with pearly luster. Model by "rocksandminerals" (Sketchfab).
Greasy Luster
Description: Looks like covered in fatty grease
Examples: Nepheline, opal, jadeite
Nepheline (white mineral) with greasy luster. Model by "rocksandminerals" (Sketchfab).
Silky Luster
Description: Fine, parallel fibers
Examples: Gypsum, malachite, serpentine, tiger's eye
Tiger's eye with silky luster. Model by WVU Volcanology and Petrology Lab (Sketchfab).
Earthy Luster
Description: dull, no reflection
Examples: kaolinite, limonite
Limonite with earthy luster. Model by Queen's University Geodesy and Geophysics Lab (Sketchfab).
Hardness
Hardness is a very useful property for identification, as a given mineral can only exhibit a narrow range of hardnesses, and since it is easily testable, this property can be used to quickly and simply minimize the number of possibilities. Hardness is also important because it helps us understand why some rocks are more or less resistant to weathering and erosion.
In 1824, the Austrian mineralogist Friedrich Mohs selected ten minerals to which all other minerals could be compared to determine their relative hardness. The scale became known as the Mohs Scale of Hardness, and it characterizes the resistance of a mineral to being scratched.
| Mohs Value | Mineral Example |
|---|---|
| 1 | Talc |
| 2 | Gypsum |
| 3 | Calcite |
| 4 | Fluorite |
| 5 | Apatite |
| 6 | Orthoclase |
| 7 | Quartz |
| 8 | Topaz |
| 9 | Corundum |
| 10 | Diamond |
Mohs scale of mineral hardness.
A score of 1 is the softest (i.e., easily scratched), while 10 is the hardest (i.e., cannot be scratched). Harder objects can scratch softer items. As an example, your fingernail (Mohs value = 2.5) can scratch gypsum (Mohs value = 2), but not calcite (Mohs value = 3). Softer objects, however, cannot scratch harder items. As an example, glass (Mohs value = 5.5) cannot scratch orthoclase (Mohs value = 6). The hardnesses of other common objects include: a copper penny (hardness 3), a wire nail (hardness 4.5), and unglazed porcelain (hardness 7).
Quartz, with a rating of 7 on the Mohs scale, is a relatively hard mineral, but the mineral calcite, rating 3 on the Mohs scale, is significantly softer. Therefore, it should be no surprise that quartz sandstone is much more resistant to erosion and weathering than is limestone, which is primarily made of calcite. Quartz is a very common mineral in the Earth's crust, and it is quite resistant due to its hardness and relative insolubility. Thus, quartz grains are the dominant mineral type in nearly all types of sand.
Cleavage
Many minerals break along zones of weakness called cleavage planes that reflect their underlying atomic structure. These flat cleavage planes give some mineral crystals distinctive shapes that are very useful for identification. The video below shows an example of what happens when the mineral calcite is struck by a hammer. Notice how the broken pieces form rhombohedrons, reflecting the fact that calcite has three cleavage planes that are not at 90°.
The major types of cleavage patterns, along with examples, are presented below.
Basal Cleavage
Number of cleavage planes: 1
Characteristic: Breaks into thin sheets
Examples: Muscovite, biotite, graphite
Muscovite. Model by Earth Sciences, University of Newcastle (Sketchfab).
Prismatic Cleavage
Number of cleavage planes: 2 (angle between two cleavage planes may be either ~90°, or not 90°)
Characteristic: Crystals form either elongate rectangles (cleavage planes at ~90°) or elongate parallelograms (cleavage planes not at 90°)
Examples: Pyroxenes (cleavage planes at ~90°; e.g., augite), amphiboles (cleavage planes not at ~90°; e.g., hornblende)
Augite (a type of pyroxene) with prismatic cleavage (planes at ~90°). Model by "rocksandminerals" (Sketchfab).
Cubic Cleavage
Number of cleavage planes: 3
Characteristic: Cleavage planes are arranged at 90°, forming cubes
Example: Halite (salt)
Halite with cubic cleavage. Model by Barbucha Studio (Sketchfab).
Rhombohedral Cleavage
Number of cleavage planes: 3
Characteristic: Cleavage planes are arranged at 90°, forming rhombohedrons
Example: Calcite
Calcite with rhombohedral cleavage. Model by Edurock - Educational Virtual Rock Collection (Sketchfab).
Octohedral Cleavage
Number of cleavage planes: 4
Characteristic: Crystals form 8-sided octohedrons
Example: Fluorite
Fluorite with octohedral cleavage. Model by Museum of Mineralogy and Petrography, UAIC (Sketchfab).
Dodecahedral Cleavage
Number of cleavage planes: 6
Characteristic: Crystals form 12-sided dodecahedrons.
Example: Sphalerite
No Cleavage (Fractures)
Not all minerals exhibit cleavage. This is especially true when the strength of a mineral's underlying atomic bonds are uniform in all directions.
Obsidian (volcanic glass) is not a mineral (it does not have a consistent crystal structure), but it fractures in characteristic conchoidal (rounded, clam shell-shaped) patterns. Human-made glass also fractures in this way.
Quartz lacks cleavage, though often exhibits a prismatic crystal habit (see below).
Obsidian with conchoidal fractures. Model by Sara Carena (Sketchfab).
Crystal Habit
Some minerals acquire distinctive shapes and forms as they grow that can help with their identification. These shapes and forms are known as their crystal habit. Crystal habit is different from cleavage because it does not correspond with planes of weakness at the atomic scale. Examples of several different types of crystal habits are shown below.
Cubic Habit
Characteristic: Minerals cube-shaped, but not due to cleavage.
Example: Pyrite (sometimes)
Sample of pyrite with a cubic habit. Model by "jadams.moz" (Sketchfab).
Prismatic Habit
Characteristic: Minerals prism-shaped.
Example: Quartz (sometimes)
Sample of smoky quartz with a prismatic habit. Model by Earth Sciences, University of Newcastle (Sketchfab).
Dodecahedral Habit
Characteristic: Minerals 12-sided, but not due to cleavage.
Example: Garnet
Sample of garnet with dodecahedral habit. Model by the Digital Atlas of Ancient Life and Paleontological Research Institution (Sketchfab).
Other Properties of Minerals
Besides color, luster, hardness, and cleavage, other properties of minerals also help aid in their identification. For example, some minerals, especially metals, are much denser than others. Finding the exact density is straightforward, but it does require measuring the volume of the sample. Placing an unknown mineral in water (or other liquid) to find its volume by displacement can be a risky undertaking since several minerals react violently with water, and many more break down with exposure. Some minerals like magnetite are magnetic (affected by magnetic fields), while a few are natural magnets (capable of producing a magnetic field).
Some minerals have rare properties. For example, there are minerals that exhibit luminescence of all types, giving off light due to a particular stimulus. Some minerals are radioactive, usually due to the inclusion of significant amounts of uranium, thorium, or potassium in their structure. Carbonate minerals will effervesce when exposed to hydrochloric acid. Double refraction describes the result of light passing through a material that splits it into two polarized sets of rays, doubling images viewed through that material. For example, a single line on a sheet of paper will appear as two parallel lines when viewed through a clear calcite crystal.
What Distinguishes a Regular Mineral from a Gem?
Minerals are assigned to the category of gemstones based primarily on our interpretation of what has value. Typically, the beauty, durability, and rarity of a mineral qualify it as a gemstone. Beauty refers to the luster, color, transparency, and brilliance of the mineral, though to some degree it is dependent on the skillfulness of the cut. Not all gems are prized for these reasons; for example, scarcity may be artificially inflated, or a mineral may be valued for its unusual color. (A recent article in the New Yorker magazine by Ed Caesar provides an overview of how the global diamond market operates, as well as the hunt for the world's largest diamonds.)
Gemstones can be further categorized as precious or semiprecious stones. Precious stones, including diamond, topaz, and sapphire, are rare and translucent to light. They are more durable because they are hard, making them scratch resistant. On the Mohs scale of hardness, the majority of precious gemstones have values greater than 7. Semi-precious stones are generally softer, with hardness scale values between 5 and 7. The minerals peridot, jade, garnet, amethyst, citrine, rose quartz, tourmaline, and turquoise are examples of semi-precious stones that can be cut and used in jewelry.
Gems may have common names that differ from their geological ones, and these names may be dependent on mineral color. For example, the mineral beryl is also referred to as emerald, aquamarine, or morganite depending on its color. Corundum can also be called sapphire or ruby, and peridot is another name for olivine.
How Do Mineral Deposits Form?
Economically recoverable mineral deposits are formed by geologic processes that can selectively concentrate desirable elements in a relatively small area. These processes may be physical or chemical, and they fall into four categories:
Magmatic Processes
Magmatic processes separate minor elements of magma from the major elements and concentrate them in a small volume of rock. This may involve either the early crystallization of ore minerals from the magma while most other components remain molten or late crystallization after most other components have crystallized. Magmatic processes responsible for the formation of mineral deposits are usually associated with igneous intrusion (formed during mountain building events, rifting, and volcanic activity), which can range in composition from granite (felsic) to gabbro (mafic). Metamorphism may also cause recrystallization of minerals and concentration of rare elements. Under conditions of extreme high-temperature metamorphism, minerals with the lowest melting temperatures in the crust may melt to form small quantities of pegmatite magmas.
Hydrothermal Processes
Hydrothermal processes involve hydrothermal solutions that dissolve minor elements dispersed through large volumes of rock, transport them to a new location, and precipitate them in a small area at a much higher concentration. Hot water enriched in salts such as sodium chloride (NaCl), potassium chloride (KCl), and calcium chloride (CaCl2) is called a hydrothermal solution, or simply "brine." The brine is as salty or even saltier than seawater, acidic, and may contain minute bits of dissolved minerals such as gold, lead, copper, and zinc. The presence of salt in the water stops the metallic minerals from precipitating out of the brine because the chlorides in the salt preferentially bond with the metals. Additionally, because the brine is hot (from over 600°C (~1100°F) to less than 60°C (140°F)), the minerals are more easily dissolved, just as hot tea dissolves sugar more easily than cold tea does.
Hot water brines can have varying origins. Most bodies of magma contain mineral-enriched, superheated water, which is released into the surrounding rock as the magma cools. Rainwater can become a hydrothermal solution as it filters through rocks and picks up soluble materials along its path. Seawater, which is already enriched in salt, often becomes a hydrothermal solution in the vicinity of volcanic activity on the ocean floor where tectonic plates are pulling apart.
Hydrothermal solutions move away from their source of heating through cracks, faults, and solution channels into the adjacent cooler rocks. Some of these fluids may travel very long distances through permeable sedimentary rock. As the water moves quickly through fractures and openings in the rock (where it experiences changes in pressure or composition and dilution with groundwater), it can cool rapidly. This rapid cooling over short distances allows concentrations of minerals to be deposited. When a hydrothermal solution cools sufficiently, the dissolved salts form a precipitate, leaving behind concentrated mineral deposits in a vein or strata-bound deposit.
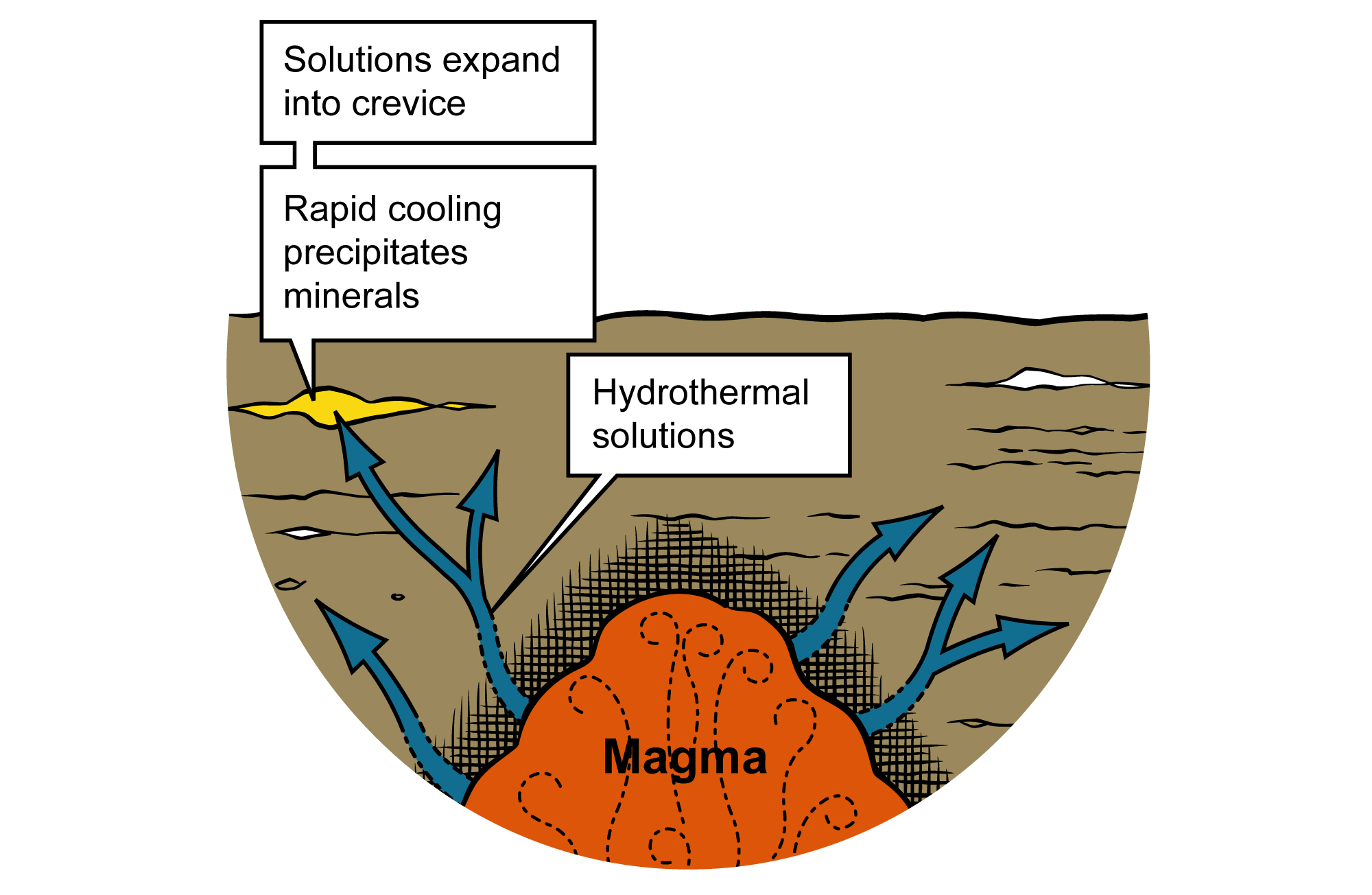
Formation of dissolved minerals from hydrothermic solutions. Image modified from original by J. Houghton, originally published in Ansley (2000).
Sedimentary Processes
Sedimentary processes gather elements dispersed through large volumes of water and precipitate them in a sedimentary environment, such as in sedimentary layers on the ocean floor or on lakebeds. Sedimentary mineral deposits form by direct precipitation from the water.
Weathering and Erosion
Weathering and erosion break down large volumes of rock by physical and chemical means and gather previously dispersed elements or minerals into highly concentrated deposits. Residual weathering deposits are mineral deposits formed through the concentration of a weathering-resistant mineral, as a result of surrounding minerals being eroded and dissolved. In contrast, mineral deposits formed by the concentration of minerals in moving waters are called placer deposits.
Classification of Minerals
The approximately 5,500 different types of minerals are classified based upon their underlying chemistry. Even though there are thousands of minerals, only 30 or so are common and abundant in Earth's crust. These are referred to as the "rock forming minerals." These are organized here into two broad groups: 1) silicate minerals; and 2) non-silicate minerals, which include native element minerals, halides, oxides, sulfides, sulfates, and carbonates.
These mineral groups are presented on the following pages, beginning with silicate minerals.
References and Further Reading
Caesar, Ed. The woman shaking up the diamond industry. The New Yorker, February 3, 2020 issue.
International Mineralogical Association. List of Minerals. Last updated January 2020.
Klein, C., and C. S. Hurlbut, Jr. 1999. Manual of Mineralogy, 21st Edition, revised. John Wiley & Sons, Inc. New York, 681 pp.
Mason, B., and C. B. Moore. 1982. Principles of Geochemistry, 4th Edition. John Wiley & Sons, Inc., New York.



