Contents
Page topics: The Silica Tetrahedron; Bowen's Reaction Series; Minerals with Isolated Tetrahedra; Single-Chain Silicates; Double-Chain Silicates; Sheet Silicates; Framework Silicates
Image above: Two examples of silicate minerals: potassium feldspar (left) and muscovite mica (right). Image created by Jonathan R. Hendricks for the Earth@Home project (CC BY-NC-SA 4.0 license).
Silicate Minerals and the Silica Tetrahedron
Silicate minerals are by far the most abundant minerals on the surface of the Earth. All silicate minerals share a common building block: the silica tetrahedron. This four-sided molecule consists of four oxygen (O) atoms and one silicon (Si) atom. The oxygen atoms are anions with a minus-2 charge (O2-) that are covalently bonded to a single silicon cation with a plus-4 charge (Si4+). Each face of the silica tetrahedron forms an equilateral triangle. As you will see below, different types of silicate minerals are characterized based on how the silica tetrahedra are bonded to each other, as well as to other elements. Because of the abundance of silicate minerals and their underlying silica tetrahedra building blocks, Si and O comprise 75% of the mass of the Earth’s crust.
Model of a silica tetrahedron [SiO₄]⁴⁻; the yellow spheres represent oxygen and the pink sphere represents silicone. Model by Earth Sciences, University of Newcastle (Sketchfab).
The four oxygen anions have a combined charge of -8 and the silicone cation has a charge of +4, giving the silica tetrahedron building block a total negative charge of -4 and a chemical equation of [SiO₄]⁴⁻. This negative charge causes the tetrahedron to have a great affinity for atoms or molecules with positive charges.
There are two common solutions to this problem of excess negative charge. One is to add more cations. The mineral olivine is formed in this way, with two magnesium cations (Mg2+) attached to each silica tetrahedron, resulting in the chemical equation Mg2SiO4.
The second way for the silica tetrahedra to balance their charges is to link to one another. The mineral quartz provides an example of this solution, where all four corners of each tetrahedron are linked to others, producing the overall mineral chemical formula SiO2.
Model of the atomic structure of silica in quartz (SiO₂); note that each silica tetrahedron is linked to four others. Model by Museum of Mineralogy and Petrography, UAIC (Sketchfab).
Most silicate minerals are constructed through a combination of tetrahedral linking and the addition of cations, for example, pyroxene (MgSiO3). The degree and manner of tetrahedral linking is important in controlling the behavior and properties of individual silicate minerals, and also provides a useful way to classify these minerals. There are five major groups of silicate minerals: isolated tetrahedra, single-chain silicates, double-chain silicates, sheet silicates, and framework silicates.
Before exploring these five groups, however, it is useful to be introduced to the close relationship between the atomic structure and properties of silicate minerals and the temperatures in which they crystallize.
Bowen's Reaction Series
Silicate minerals are formed from the crystallization of magma. In the early 1900s, a geologist named Norman L. Bowen (1887-1956) developed a model, later called "Bowen's reaction series," that characterized the order in which silicate minerals form as magma cools. This model is shown in the figure below.
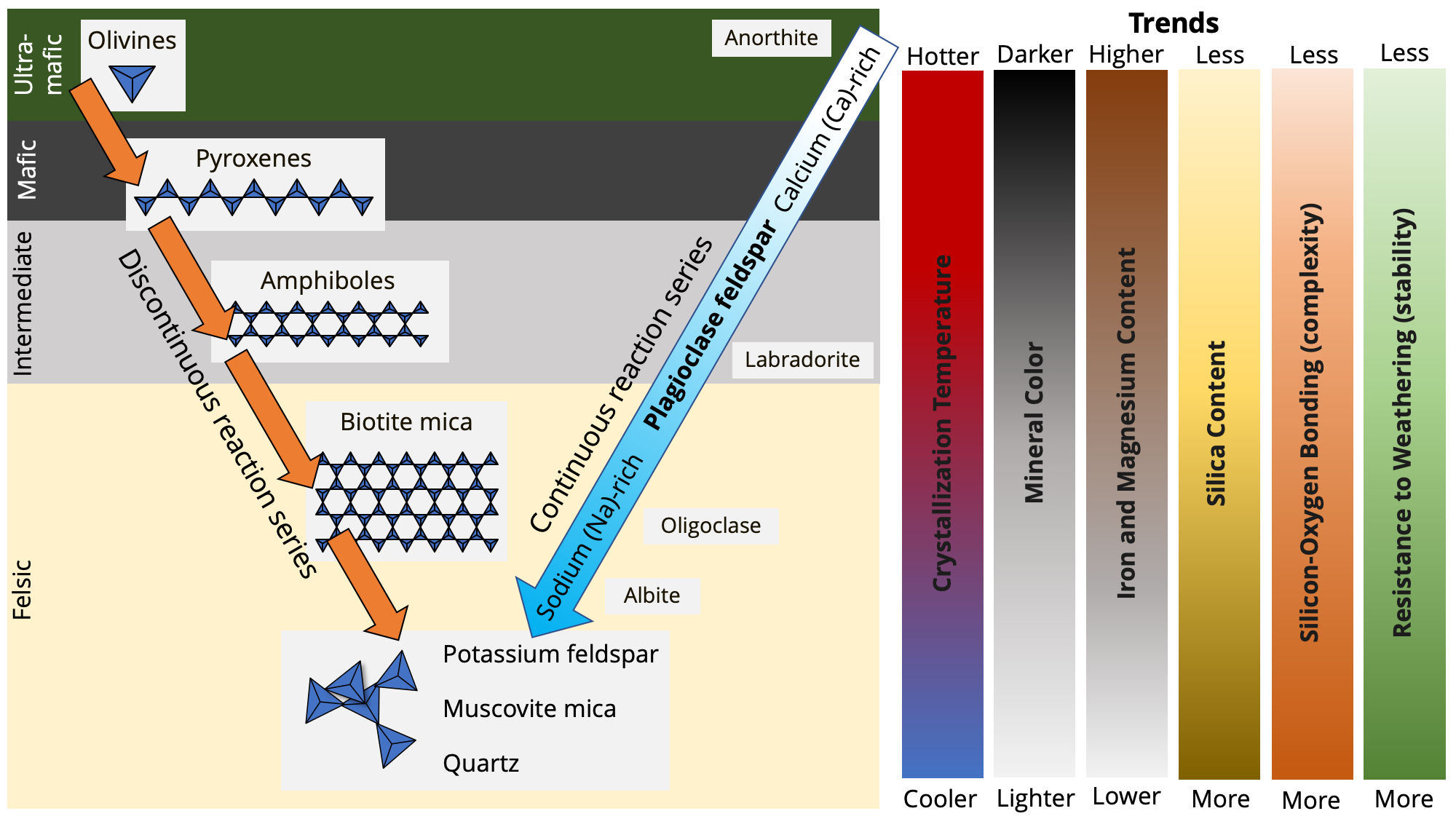
Bowen's reaction series. The left-hand trajectory is called the discontinuous series, as each mineral group is distinct; triangles represent different patterns of bonding of silica tetrahedra. The right-hand trajectory following Ca-plagioclase is the continuous series, as there is a complete transition between the Ca-rich and Na-rich plagioclase feldspars; anorthite and albite are, respectively, the Ca-rich and Na-rich end members of this series. Trends as from the top of the series to the bottom are documented on the right side of the figure; note that other than crystallization temperature, most of these trends apply to the discontinuous reaction series, rather than the continuous series. Image created by Jonathan R. Hendricks for the Earth@Home project (CC BY-NC-SA 4.0 license).
Imagine that you could look inside a chamber deep inside the Earth that is full of magma. As that magma cools, certain minerals will form crystals in a predictive, orderly fashion, which is explained by Bowen's reaction series. Different minerals have different crystallization temperatures: some crystallize out of magma at incredibly high temperatures, while others crystallize at much lower temperatures. For example, the olivine mineral forsterite crystallizes out of magma at temperatures near 1800 ºC, while pyroxenes do not form until the magma temperature reaches 1557 ºC. Further, the temperatures at which some minerals crystalize depends upon the initial chemical composition of the magma itself.
Central to Bowen's model is the concept of fractional crystallization, which dictates that minerals are removed from the cooling magma as they crystalize, for example by sinking to the bottom of a magma chamber. As a consequence, the chemical composition of the magma body changes as it cools because some components of its initial composition become sequestered away in minerals as they crystallize and are "removed" from the liquid magma.
The types of minerals (and therefore rocks) formed from a body of cooling magma is ultimately a consequence of the chemistry of the magma itself. For example, magmas that have relatively low amounts of silica will produce minerals like olivines, pyroxenes, and amphiboles as they cool, but virtually no quartz. These minerals are dark in color and the resulting rocks are characterized as mafic. Alternatively, magmas that are rich in silica produce minerals such as quartz and potassium feldspar, but not minerals like olivine. Quartz and potassium feldspar are light in color and rocks that are rich in these minerals are characterized as felsic.
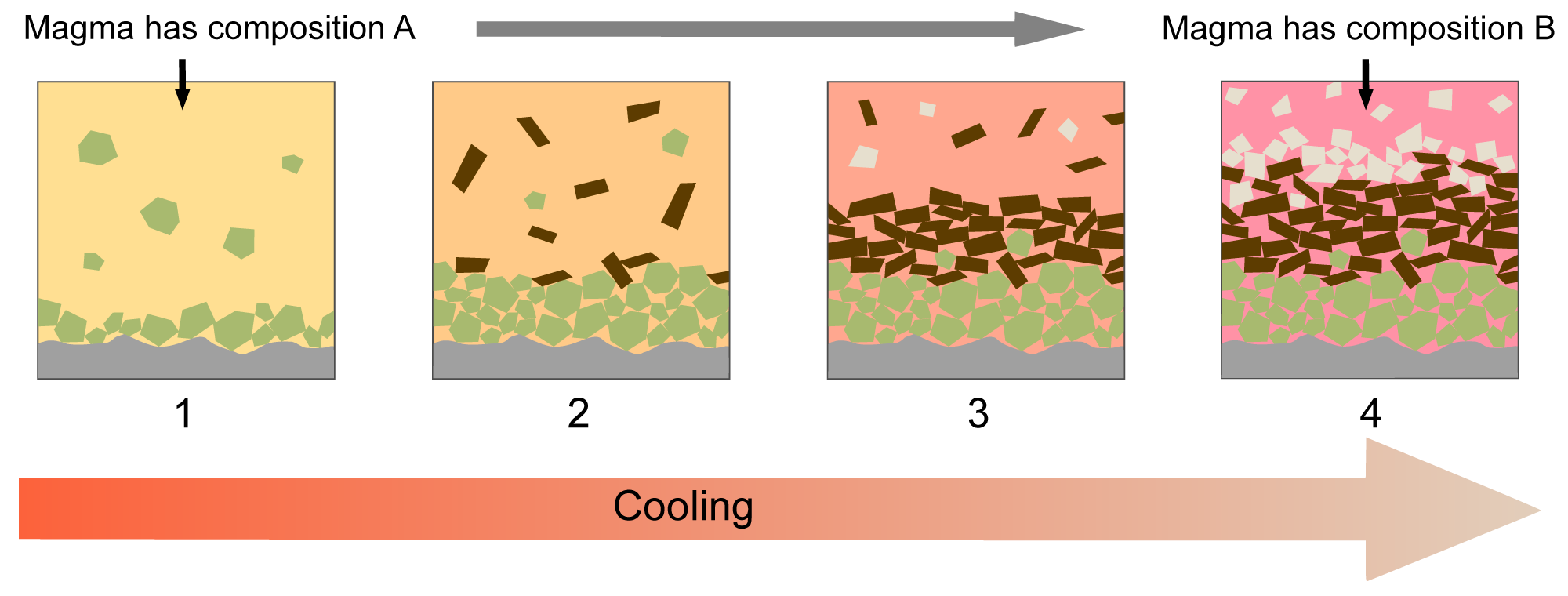
Illustration of the process of fractional crystallization. Modified from original image by "Woudloper" (Wikimedia Commons; Creative Commons Attribution-Share Alike 3.0 Unported license). Original caption: "Schematic diagrams showing the principles behind fractional crystallisation in a magma. While cooling, the magma evolves in composition because different minerals crystallize from the melt. 1: olivine crystallizes; 2: olivine and pyroxene crystallize; 3: pyroxene and plagioclase crystallize; 4: plagioclase crystallizes. At the bottom of the magma reservoir, a cumulate rock forms."
Bowen's model has two component series. The first is a continuous series describes the formation of plagioclase feldspar crystals that become increasingly enriched in sodium and depleted in calcium. The second is a discontinuous series that describes the successive formation of minerals belonging to the olivine, pyroxene, and amphibole groups, as well as biotite mica. Note that the crystallization of minerals in the continuous and discontinuous series happens simultaneously.
Continuous Reaction Series
The continuous reaction series in Bowen's model explains the formation of different types of plagioclase feldspar (geologists often refer to members of this mineral group as "plag," pronounced with a "g" as in George). In members of this mineral group, atoms of calcium and sodium can substitute for each other, with the relative percentages of Ca and Na defining individual minerals, without any important changes in the underlying crystal structure. As a body of magma begins to cool, the first plagioclase feldspar minerals to crystallize are enriched in calcium (Ca). The purest endmember is a mineral called anorthite (CaAl2Si2O8), which has no sodium. As cooling proceeds, the magma becomes depleted in calcium (because it is "locked away" in minerals like anorthite) and the plagioclase feldspar minerals formed become enriched instead in sodium (Na). Some individual crystal grains of plagioclase feldspar record this history with centers that are rich in calcium and outer zones with greater amounts of sodium.The endmember mineral albite (NaAlSi3O8) has almost no calcium, just as anorthite has virtually no sodium. Intermediate member minerals of the plagioclase feldspar series include oligoclase and the lovely mineral labradorite.
Discontinuous Reaction Series
The discontinuous reaction series in Bowen's model describes the crystallization of distinct types of minerals as a body of magma cools. Members of the olivine group have the highest crystallization temperature (~1200–1800 °C), so are the first to crystallize out of a silica-poor magma and sink to the bottom of the magma chamber. (A different way to think about this is that olivines have the highest melting temperature.) As the magma continues to cool, pyroxenes are the next to crystallize, followed by amphiboles and then biotite mica.
Potassium Feldspar, Muscovite Mica, and Quartz
Members of the potassium feldspar group (commonly also called "K-feldspar," "K-spar," or "alkali feldspar"); orthoclase is an example), muscovite mica, and quartz are three of the final minerals that might crystallize from a cooling body of magma once it reaches a temperature of around 600 ºC, but only if that magma is rich in silica. These minerals are indicative of granite, which also includes albite, which is sodium-rich potassium feldspar.
Trends Associated with Bowen's Reaction Series
The elegance of Bowen’s Reaction Series (BRS) lies in its ability to explain an array of seemingly disparate aspects of silicate minerals and the rocks that they comprise. As one moves from the top of Bowen's model to the bottom, a handful of trends become apparent, some of which have already been alluded to previously (compare with figure of Bowen's model above). Note that other than crystallization temperature, most of these trends apply to the discontinuous reaction series, rather than the continuous series.
Crystallization temperature: Minerals higher in BRS have higher crystallization temperatures than those that are lower in BRS. For example, olivine minerals crystallize at a much higher temperature than quartz. (Alternative, quartz melts at a much lower temperature than olivine).
Mineral color: In general, minerals higher in the discontinuous series of BRS are darker in color than those that are lower in BRS. For example, pyroxene is darker in color than potassium feldspar. Rocks that are enriched in dark-colored minerals like olivine and pyroxene are mafic, while those that are enriched in light-colored minerals like potassium feldspar and quartz are felsic.
Iron and magnesium content: Minerals that are higher in the discontinuous series of BRS have greater amounts of iron (Fe) and magnesium (Mg) than those that are lower in the series.
Silica content: Minerals that are lower in the discontinuous series of BRS have greater amounts of silica than those that are higher in the series.
Silicon-oxygen bonding (complexity): Minerals that are lower in the discontinuous series of BRS have greater amounts of silica tetrahedra bonding than those that are higher in the series (see more below). This makes minerals that are lower in the series more resistant to weathering than those that are higher in the series.
Resistance to weathering (stability): Minerals that are lower in the discontinuous series of BRS are more resistant to weathering than those that are higher in the series. This greater resistance corresponds to their increased degree of silica tetrahedral bonding.
Minerals with Isolated Tetrahedra (Nesosilicates)
In this class of silicate minerals, the tetrahedra anions ([SiO₄]⁴⁻) are connected to cations, rather than each other. Olivines, mentioned above, are a group of minerals that have this structure. One type of olivine is forsterite. Its chemical formula, Mg2SiO4, demonstrates that the silica tetrahedra are bonded to magnesium (Mg); in particular, each tetrahedron shares four Mg2+ cations. The atomic-level crystal structure of forsterite is shown in the interactive model below. Note in the model that the silica tetrahedra (shaded white) do not connect directly with each other; instead, they are isolated.
Atomic-level crystal structure of the mineral forsterite (Mg2SiO4). Model by Museum of Mineralogy and Petrography, UAIC (Sketchfab).
Fayalite is the other common mineral in the olivine group. In this mineral, the silica tetrahedra are bonded to iron cations (Fe2+) rather than magnesium, resulting in the chemical formula Fe2SiO4. One result of the insular tetrahedral structure of olivine minerals is the relatively low Si to O ratio of 1:4.
Example: Olivine Group
Formula: Mg2SiO4 (forsterite); Fe2SiO4 (fayalite)
Properties: Green color, glassy luster, forms small grains rather than large crystals
Abundant in these rocks: Peridotite, dunite
Trivia: Much of Earth's mantle consists of olivine; Hawaii's green sand beach is composed largely of olivine.
Sample of peridotite xenolith containing abundant olivine (green mineral). Model by "phaneritic" (Sketchfab).
Single-Chain Silicates (Inosilicates)
What if we connect each silica tetrahedron to two others? This arrangement would form a chain, the basis for the single-chain silicates, which are also known as inosilicates (Greek, inos = fiber). Minerals in the pyroxene group are constructed from a single chain of silica tetrahedra, where two corners of each tetrahedron are linked to neighbors in an infinite chain.
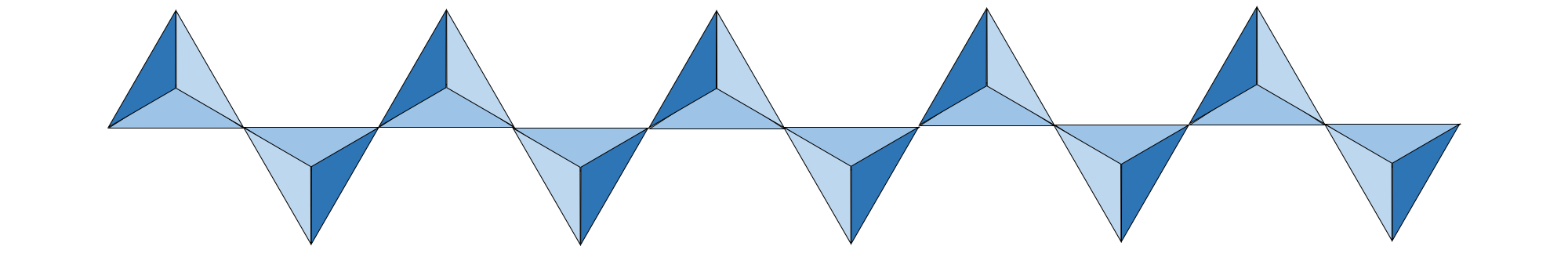
Illustration of a single chain of silica tetrahedra; each tetrahedron is connected to two other tetrahedra. Image created by Jonathan R. Hendricks for the Earth@Home project (CC BY-NC-SA 4.0 license).
Atomic-level crystal structure of the single-chain silicate mineral diopside. Silica tetrahedra are dark blue, magnesium cations are light blue, and calcium cations are orange. Model by Earth Sciences, University of New Castle (Sketchfab).
This gives pyroxene a Si:O ratio of 1:3. Cations such as Mg, Ca, Fe, and Al are still required to maintain charge balance. The pyroxene minerals crystallize at lower temperatures than olivine (< 1400℃), but still higher than most minerals. There are two major subgroups of single-chain silicates that are defined based on their crystal structures. One of these subgroups is the clinopyroxenes (augite and diopside are examples); the other is the orthopyroxenes (enstatite is an example).
Example: Augite (Pyroxene Group)
Formula: (Ca,Na)(Mg,Fe,Al)(Si,Al)₂O₆ (Note: elements separated by commas in the equation may substitute for each other.)
Properties: Black, vitreous luster
Abundant in these rocks: Peridotite, basalt, gabbro
Trivia: Augite is very common in dark-colored igneous rocks, including basalt samples collected from the surface of the moon.
Double-Chain Silicates (Inosilicates)
In double-chain silicates (which are also inosilicates), the atomic structure is defined by pairs of parallel silica tetrahedra chains, wherein every other tetrahedron shares an oxygen.
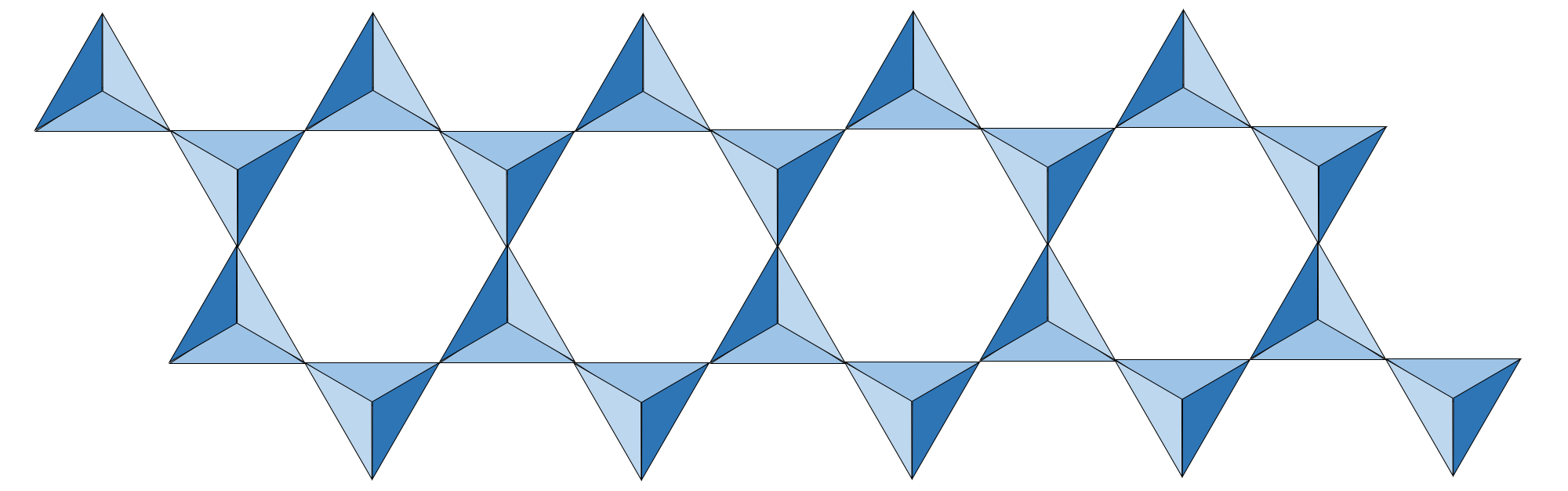
Illustration of a double chain of silica tetrahedra. Note that every other silica tetrahedron shares a corner oxygen with the parallel chain. Image created by Jonathan R. Hendricks for the Earth@Home project (CC BY-NC-SA 4.0 license).
Atomic-level crystal structure of the double-chain silicate mineral hornblende. Silica tetrahedra are dark blue, iron and magnesium cations are orange, and calcium cations are light blue. Model by Earth Sciences, University of New Castle (Sketchfab).
The amphibole silicate mineral group has this double-chain silica tetrahedral atomic structure. The name amphibole derives from the Greek word for “ambiguous,” as amphibole minerals span a wide range of physical appearance. Amphibole chemistry includes an OH- anion, along with common cations Mg, Ca, Fe, Al, Na. The Si:O ratio in amphibole is 8:22 (1:2¾).
Example: Hornblende (Amphibole Group)
Formula: (Ca,Na)₂₋₃(Mg,Fe,Al)₅Si₆(Si,Al)₂O₂₂(OH)₂ (Note: elements separated by commas in the equation may substitute for each other.)
Properties: Dark green, vitreous luster, fibrous
Abundant in this rock: Diorite
The amphibole mineral hornblende, a type of amphibole. Model by Nate Siddle for The University of Queensland School of Earth and Environmental Sciences (Sketchfab).
Sheet Silicates (Phyllosilicates)
In sheet silicates, also called phyllosilicates (Greek, phyllon=leaf), each tetrahedron shares three corner oxygen atoms with its neighbors.
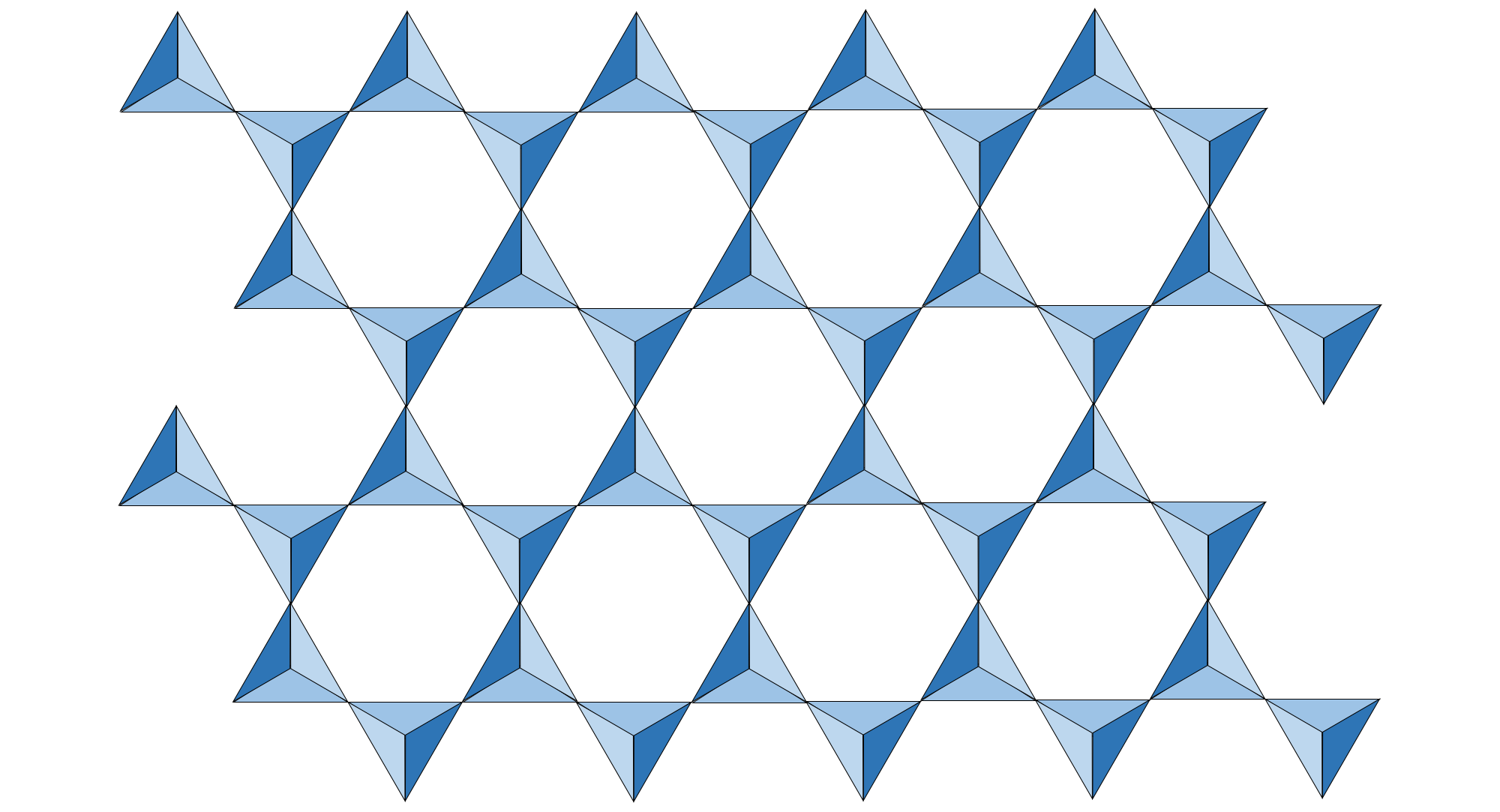
Illustration of a sheet of silica tetrahedra. Note that each silica tetrahedron shares a corner oxygen with three other tetrahedra. Image created by Jonathan R. Hendricks for the Earth@Home project (CC BY-NC-SA 4.0 license).
Atomic structure of the sheet silicate muscovite, a type of mica. Silica tetrahedra are dark blue, alumina are light blue, and potassium ions are purple. Model by Earth Sciences, University of Newcastle (Sketchfab).
Mica group (e.g., biotite and muscovite) minerals have this atomic structure, giving mica a Si:O ratio of 4:10 (1:2½). The internal arrangement of tetrahedra is expressed at the macroscopic level as well, as all mica minerals are characterized by a flaky sheet-like structure. Mica chemistry includes the OH- anion, common cations Mg, Ca, Fe, Al, Na, and in some forms also K, Li, Ti, Cr, Mn. Mica is a widely used commercial mineral group, in applications from electronics to construction to cosmetics. In addition to micas, clay minerals such as kaolinite and talc are also examples of sheet silicates, as are some asbestos minerals such as chrysotile.
Example: Biotite (Mica Group)
Formula: K(Mg,Fe)₃(AlSi₃O₁₀)(OH)₂ (Note: elements separated by commas in the equation may substitute for each other.)
Properties: Brown to black, cleaves into thin sheets
Abundant in this rocks: Granites, diorites
A sample of biotite mica, a type of sheet silicate. Model by "rocksandminerals" (Sketchfab).
Framework Silicates (Tectosilicates)
In the framework, or tectosilicates (Greek, téktōn=builder, carpenter), group, every oxygen atom in every tetrahedron is shared with another, forming a three-dimensional framework.
Atomic structure of the framework silicate quartz. Note that each tetrahedron is linked to four other tetrahedra. Model by Earth Sciences, University of Newcastle (Sketchfab).
The charge imbalance is reduced to zero, thus no additional cations are required. This arrangement is best represented by quartz, with formula SiO2. Clearly, quartz has a Si:O ratio of 1:2. Pure quartz is colorless, but common impurities produce quartz crystals in a variety of colors (e.g., purple amethyst, pink rose quartz, yellow citrine). An additional consequence of the 3D arrangement of tetrahedra is that quartz crystals form in the shape of hexagonal prisms. The Si:O ratio that we have been tracking through the crystallization sequence is a simple metric for the degree of tetrahedral linking—or polymerization—of the crystal structure. This polymerization is very important for the subsequent behavior of the silicate minerals and the rocks that they form.
Feldspars are an exception to the orderly progression of tetrahedral linking that we have encountered so far. Like quartz, all feldspars are tectosilicates. Unlike quartz, however, they are not exclusively frameworks of silica tetrahedra. Feldspar minerals include aluminum in their crystal structure along with Ca, Na, and K cations. Orthoclase feldspar (KAlSi3O8), a type of potassium feldspar, is part of the discontinuous series, with a relatively low crystallization temperature that falls between muscovite mica and quartz. As noted above, Ca-plagioclase and Na-plagioclase form a continuous series. They are a solid solution, where there is continuous variation between the two end-member compositions: 100% Ca-plagioclase (anorthite, with 0% Na) to 100% Na-plagioclase (albite, with 0% Ca), passing through every possible combination in between. Calcium plagioclase crystallizes at high temperature, while sodium plagioclase crystallizes at a lower temperature. The intermediate compositions are given their own names, including bytownite, labradorite, andesine, and oligoclase.
Example: Quartz
Formula: SiO₂
Properties: Colorless or white, unless impurities are present; transparent, hardness of 7
Abundant in these rocks: Siliciclastic sedimentary rocks (e.g., sandstone, siltstone) often have large amounts of quartz derived from igneous rocks such as granites and diorites.
Sample of amethyst quartz, a framework silicate. Model by "Panomedia" (Sketchfab).
Example: Orthoclase (Potassium Feldspar Group)
Formula: KAlSi3O8
Properties: White, gray, or pink; luster vitreous; right-angle cleavage present
Abundant in this rocks: Granites, rhyolites
Sample of potassium feldspar (orthoclase), a framework silicate. Model by Paleontological Research Institution (Sketchfab).
Summary
The systematic changes in temperature and structure defined by Bowen’s reaction series lead to systematic changes in the properties, behavior, and occurrence of these silicate minerals, and the rocks that they form. Among the most important of these is the resistance of different minerals to chemical or mechanical attack. Minerals with less tetrahedral linking (e.g., nesosilicates and inosilicates) are more susceptible to weathering at the Earth’s surface, while the highly polymerized minerals are more resistant to weathering. The degree of polymerization also controls the behavior of liquid silicate magma and the different eruptive behaviors exhibited by magmas.
There are other important mineral groups in addition to the silicates that comprise Bowen’s reaction series, including—for example—carbonates, sulfides, oxides, and native elements. These will be briefly introduced on the next page.
References and Further Reading
Klein, C., and C. S. Hurlbut, Jr. 1999. Manual of Mineralogy, after J.D. Dana. John Wiley & Sons, Inc., New York, 681 pp.
Press, F., and R. Siever. 1994. Understanding Earth. W. H. Freeman and Company, New York, 593 pp.



